Have you ever wondered how the solar panels you see on rooftops and in fields can turn sunlight into electricity? It might seem like magic, but it’s actually all about science! Let’s explore how solar panels work, step by step, and delve into the fascinating history of the photovoltaic effect.
The Discovery of the Photovoltaic Effect
The photovoltaic effect, the process that allows solar panels to convert sunlight into electricity, has an interesting history. It was first discovered by a French physicist named Alexandre Edmond Becquerel in 1839. While experimenting with an electrolytic cell made up of two metal electrodes placed in an electrolyte solution, he noticed that when exposed to light, the cell produced more electricity. This was the first observation of the photovoltaic effect.
Early Developments
In 1873, Willoughby Smith discovered the photoconductivity of selenium. Later, in 1876, William Grylls Adams and Richard Evans Day observed that selenium produces electricity when exposed to light. This was a significant step toward developing practical solar cells.
Albert Einstein’s Contribution
In 1905, Albert Einstein published a groundbreaking paper explaining the photoelectric effect, which is closely related to the photovoltaic effect. He proposed that light could be thought of as packets of energy called photons. When these photons strike a material, they can transfer enough energy to electrons to free them from their atoms. This explanation of the photoelectric effect provided a deeper understanding of how light interacts with materials to generate electricity. For this work, Einstein was awarded the Nobel Prize in Physics in 1921.
Practical Solar Cells
The first actual solar cell, capable of converting sunlight into electricity, was created in 1954 by Bell Laboratories. Scientists Daryl Chapin, Calvin Fuller, and Gerald Pearson developed a silicon-based solar cell that had an efficiency of 6%, which was a breakthrough at the time.
How Does the Photovoltaic Effect Work?
Absorbing Sunlight:
When sunlight shines on a solar cell, it hits the silicon material. Sunlight is made up of tiny particles called photons. When these photons strike the silicon, they transfer their energy to electrons in the silicon atoms.
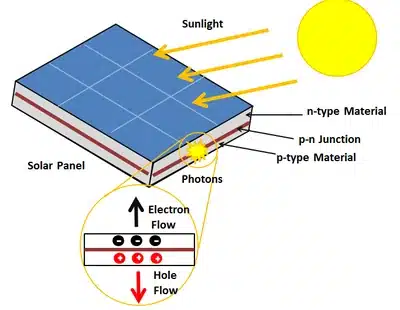
Creating Electron-Hole Pairs:
The energy from the photons excites the electrons, giving them enough energy to break free from their atoms. When an electron leaves its atom, it leaves behind a “hole.” This hole is simply the absence of an electron, and it acts like a positive charge while the electron is a negative charge. Together, these are called electron-hole pairs.
The Role of the P-N Junction:
Inside the solar cell, there’s a special region where two different types of silicon meet. One side is called the p-type, which has extra holes (positive charge), and the other side is the n-type, which has extra electrons (negative charge). This creates an electric field at the boundary, known as the p-n junction.
Separation of Charges:
The electric field at the p-n junction acts like a one-way gate. It pushes electrons towards the n-type side and holes towards the p-type side. This separation of charges creates a flow of electric current when the solar cell is connected to an external circuit.
Generating Electricity:
Once the electrons and holes are separated, they move through the solar cell and into an external circuit. The movement of electrons through the circuit creates an electric current. This current, along with the voltage created by the electric field, generates electrical power.
Putting It All Together: The Solar Panel
A single solar cell doesn’t produce much electricity, so many cells are connected together to form a solar panel. When sunlight hits the panel, each cell generates a small amount of electricity, and all these small amounts add up to a useful amount of power.
Solar cells are typically arranged in a grid pattern within a solar panel. The most common configurations are 60-cell and 72-cell panels. These cells are interconnected using thin metal wires known as busbars and fingers. The busbars collect the electric current generated by each cell, while the fingers distribute the current within the cell.
Series and Parallel Connections
The solar cells in a panel are connected in series and parallel combinations.
- Series Connection: In a series connection, the positive terminal of one cell is connected to the negative terminal of the next cell. This arrangement increases the voltage output of the panel while keeping the current constant. For example, if each cell produces 0.5 volts and there are 36 cells in series, the total voltage will be 18 volts.
- Parallel Connection: In a parallel connection, the positive terminals of all cells are connected together, and the negative terminals are connected together. This increases the current output while keeping the voltage constant. Parallel connections are often used to combine multiple series strings of cells.
Maximizing Efficiency
To maximize efficiency, solar panels are designed to minimize resistance and heat loss. The use of anti-reflective coatings, high-quality materials, and precise manufacturing techniques ensures that as much sunlight as possible is converted into electricity.
Output and Applications
The combined output of the interconnected solar cells generates enough power for various applications, from residential rooftops to large solar farms. By working together, solar cells in a panel efficiently capture and convert solar energy into usable electrical power, contributing to clean and renewable energy solutions.
Last words
Solar panels are a fantastic example of how science and technology can work together to solve real-world problems. By understanding the photovoltaic effect and the role of semiconductors like silicon, we can appreciate how sunlight is transformed into electricity. Solar panels not only provide a clean and renewable source of energy but also offer a way to reduce our carbon footprint and move towards a more sustainable future.
Next time you see a solar panel, you’ll know the amazing process happening inside: sunlight hitting the silicon, exciting electrons, creating electron-hole pairs, and generating electricity through the magic of the photovoltaic effect!











