Photoluminescence (PL analysis), a type of cold luminescence, refers to the process by which a substance absorbs a photon (or electromagnetic wave) and then re-radiates the photon (or electromagnetic wave).
What is PL analysis?
PL analysis is a non-destructive and highly sensitive analytical method that can provide information about the structure, composition, and atomic arrangement of a material’s environment. The use of lasers has enabled this type of analysis to penetrate the field of micro-regions, selective excitation, and transient processes, making it a further important means of research in the fields of physics, materials science, chemistry, and molecular biology, and in the manufacture of photovoltaic products, such as solar panels, to play a product quality inspection role.
What is the principle of photoluminescence & PL analysis?
From the quantum mechanical theory, this process can be described as a material absorbing photons and leaping to a higher energy level of the excited state and then returning to a lower energy state, while emitting photons. It roughly passes through three main stages: absorption, energy transfer, and light emission. Light absorption and emission both occur as a result of a jump between energy levels, all through the excited state. Energy transfer, on the other hand, is due to the motion of the excited state. Photoluminescence can be caused by ultraviolet radiation, visible light, and infrared radiation. Photoluminescence can be divided into fluorescence and phosphorescence according to the delay time.
In a typical direct bandgap photoluminescence experiment, when the energy of the incident laser photon is higher than the bandgap energy of the material itself, the semiconductor will be excited, and the incident photon will be absorbed, and at the same time in the top of the conduction band and the bottom of the valence band, respectively, the formation of electrons and holes. At this time these excited electrons and holes can not stabilize the existence of the corresponding energy level to stay for a very short period, the electron will be toward the valence band minimum (i.e., eigenstates) for the energy and momentum of the relaxation, and ultimately and the hole composite, and once again released photons.
For typical indirect bandgap semiconductor materials, the excited electrons and holes are not in the same momentum space, in the relaxation process, the electrons to the ground state for the leap, due to the mismatch in momentum space, the electrons will interact with the phonons in the lattice to further leap, and finally return to the ground state and the holes for the composite luminescence in a non-stop leap, in which the photon will be consumed in the process of the leap A large amount of energy in this process will consume photons, and converted into other types of energy, mainly thermal energy, which is typical of direct bandgap semiconductors and indirect bandgap semiconductors photoluminescence process.
What is PL intensity?
PL intensity typically refers to Photoluminescence intensity. Photoluminescence is a phenomenon where a material absorbs photons (usually from a light source) and then re-emits them as lower-energy photons. This emission of light is often used in various scientific and technical applications to study the properties of materials.
PL intensity specifically refers to the amount of light emitted by a material during photoluminescence. It is a measure of how bright or intense the emitted light is and is typically quantified in terms of units like lumens or watts per unit area or unit solid angle.
Photoluminescence is commonly used in fields such as materials science, chemistry, physics, and engineering for various purposes, including the characterization of semiconductor materials, the study of fluorescent compounds, and the investigation of the electronic properties of materials. The intensity of the emitted light can provide valuable information about the material’s composition, structure, and electronic properties.
The Pros and Cons of PL analysis
What is the difference between photoluminescence and fluorescence?
Photoluminescence and fluorescence are both processes by which matter emits light, but they differ in their underlying mechanisms and the conditions that drive the emission of light. The following are their main differences:
Mechanism of luminescence
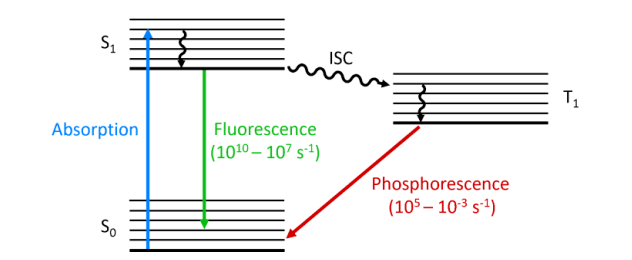
- Photoluminescence: Photoluminescence is a broad term that refers to when a material absorbs a photon (light or other electromagnetic radiation) and re-emits the observed light after a short delay. This delay can be a fraction of a second or on a much longer time scale. Photoluminescence includes fluorescence and phosphorescence.
- Fluorescence: Fluorescence is a specific type of photoluminescence. A material absorbs a photon (usually ultraviolet or visible light) and then rapidly emits a lower energy (i.e., longer wavelength) photon as a result of electron leaps within the material. Light emission in fluorescence is almost instantaneous, usually occurring within nanoseconds.
Energy states involved
- Photoluminescence: In photoluminescence, the material can return to the ground state (lowest energy state) either rapidly (fluorescence) or with a long delay (phosphorescence). The key difference is the duration of the excited state. Some materials may exhibit both fluorescence and phosphorescence, depending on the particular energy level involved.
- Fluorescence: In fluorescence, light is emitted when an excited electron in a material returns to the ground state by emitting a photon. This transition usually involves a single-electron excited state, which is more energetic and shorter-lived than a three-electron excited state (common in phosphorescence).
Luminescence duration
- Photoluminescence: Photoluminescence includes short-lived fluorescence and long-lived phosphorescence. The luminescence duration of photoluminescence depends on the energy states involved and the material properties.
- Fluorescence: Fluorescence is characterized by very short emission times, usually in the nanosecond range. It is a fast and reversible process.
Examples are given
- Photoluminescence: This term encompasses a variety of phenomena other than fluorescence, such as phosphorescence, chemiluminescence (light emission from chemical reactions), and bioluminescence (light emission from organisms such as fireflies).
- Fluorescence: Examples of fluorescence include fluorescent dyes, certain minerals, and many biomolecules, such as the green fluorescent protein (GFP) used in molecular biology research.
While photoluminescence is the general term describing the emission of light when a substance absorbs a photon, fluorescence is a specific type of photoluminescence characterized by the rapid emission of photons of lower energy. The key factors that distinguish them are the duration of the light emission, the energy states involved, and the specific conditions under which it occurs.
Related Information
In addition to luminescent PL analysis, EL inspection is also the most common solar cell quality testing method.
You can poke the following article to learn more about product reliability testing.